
If the proliferative phase is impaired or prolonged, ongoing inflammation and fibroblast proliferation impair alveolar clearance and functional recovery. This involves neutrophil apoptosis and removal, expansion of resident fibroblasts and interstitial matrix reformation, and regrowth of alveolar epithelium by differentiation of type II alveolar cells into type I cells. In the proliferative phase of ARDS, with the clearance of pathogens and damaged host cells from the alveolar space, the immune response is recalibrated to prioritize repair and restoration of normal function. 4 As a result of loss of the normal low permeability characteristics, the alveolar space fills with an inflammatory cell-rich proteinaceous edema fluid (exudative phase of ARDS), a prime determinant of lung injury severity, alveolar collapse, and derecruitment.
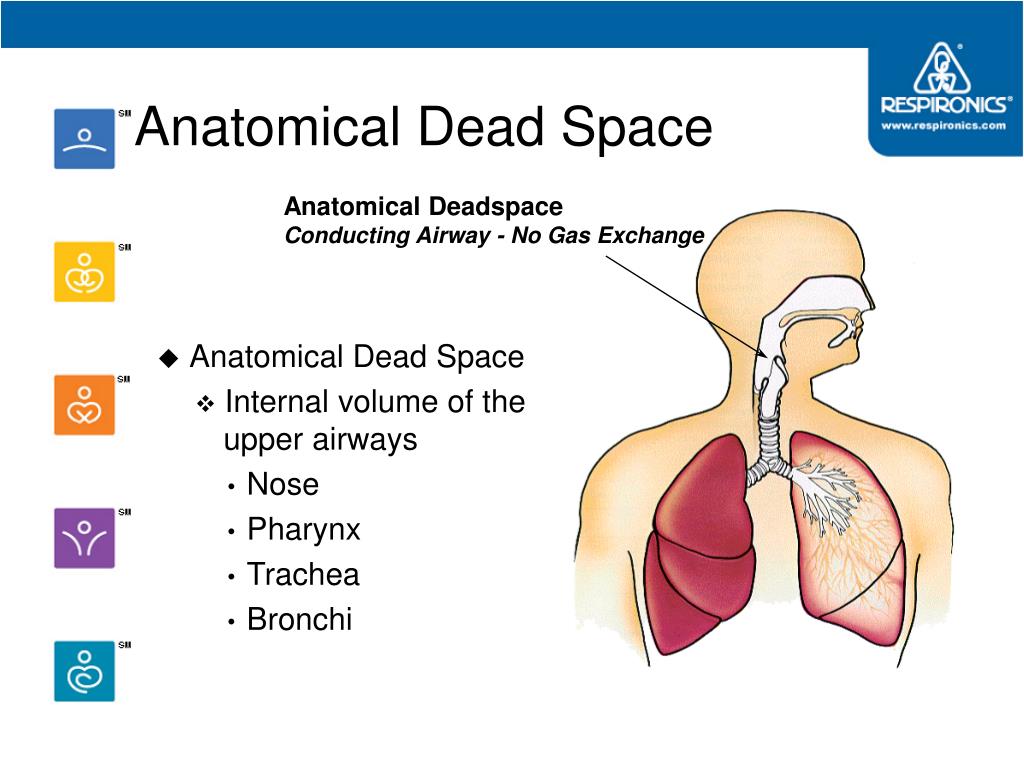
3 Alveolar type II pneumocytes secrete surfactant and along with type I pneumocytes reabsorb alveolar fluid by active ion transport back into the interstitium for lymphatic clearance.

Although assisting with pathogen killing, they also injure the normally tight alveolar endothelial–epithelial barrier consisting of adherent cell-cell contacts and glycocalyx linings. Cytokine/chemokine release by macrophages recruits and activates circulating neutrophils, which release myriad inflammatory molecules. Injury begins with activation of alveolar macrophages by microbial or cell injury products, 2 which are locally derived in primary lung injury (pulmonary ARDS) or systemically derived (extrapulmonary ARDS). It is useful to briefly review the pathogenesis of lung injury and repair in ARDS to understand its effects on physiology. Pathogenesis of Acute Respiratory Distress Syndrome


 0 kommentar(er)
0 kommentar(er)
